The Regeneration Of ______ Under ______ Conditions Is Called Fermentation.
circlemeld.com
Sep 10, 2025 · 6 min read
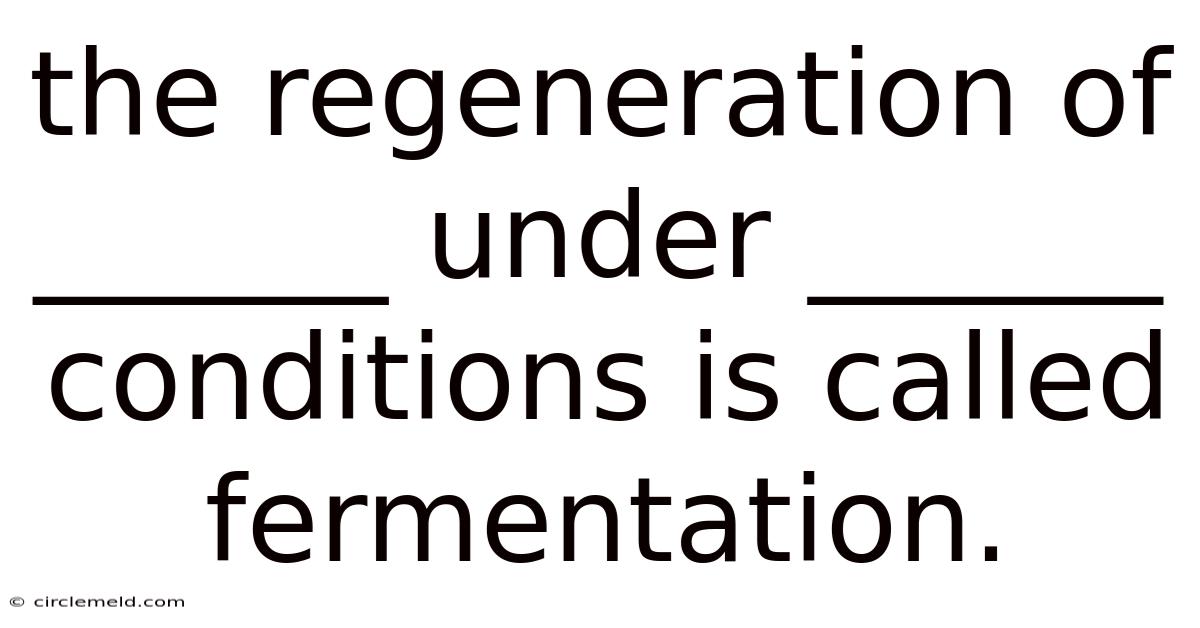
Table of Contents
The Regeneration of NAD+ Under Anaerobic Conditions is Called Fermentation
Fermentation, a process central to both microbiology and industrial biotechnology, is often misunderstood as simply the production of alcoholic beverages. While alcoholic fermentation is a prominent example, the true significance of fermentation lies in its role as an essential metabolic pathway for many organisms, allowing them to regenerate crucial electron carriers like NAD+ under anaerobic conditions – conditions lacking molecular oxygen. This regeneration is critical for continued energy production, even in the absence of respiration. This article delves into the intricacies of fermentation, exploring its mechanisms, various types, industrial applications, and its crucial role in the survival and metabolic flexibility of diverse organisms.
Introduction: The NAD+ Regeneration Conundrum
Cellular respiration, the process by which organisms extract energy from glucose and other organic molecules, relies heavily on the availability of oxygen as the final electron acceptor in the electron transport chain. This chain generates a proton gradient, which drives ATP synthesis through chemiosmosis. However, a crucial coenzyme, nicotinamide adenine dinucleotide (NAD+), plays a vital role in glycolysis, the initial stage of respiration, by accepting electrons and becoming reduced to NADH. For glycolysis to continue, NADH must be re-oxidized back to NAD+. In aerobic respiration, this re-oxidation occurs readily through the electron transport chain.
But what happens in the absence of oxygen? This is where fermentation steps in. Fermentation is essentially a metabolic pathway that allows the regeneration of NAD+ from NADH without the involvement of an external electron acceptor like oxygen. This regeneration is crucial because the accumulation of NADH would halt glycolysis, effectively stopping ATP production, ultimately leading to cell death. Thus, fermentation is an anaerobic process that sustains energy production in the absence of oxygen, albeit with significantly lower ATP yield compared to aerobic respiration.
The Steps of Fermentation: A Closer Look
Fermentation pathways are diverse, varying significantly depending on the organism and the end-products produced. However, they all share a common thread: the re-oxidation of NADH to NAD+ through a series of redox reactions. While the specific reactions differ, the core principle remains the same. Let's examine some key steps:
-
Glycolysis: The starting point of fermentation is always glycolysis. This is a series of ten enzyme-catalyzed reactions that convert one molecule of glucose into two molecules of pyruvate. During glycolysis, two molecules of NAD+ are reduced to two molecules of NADH. This step generates a net gain of 2 ATP molecules.
-
Pyruvate Reduction: The fate of pyruvate depends on the specific type of fermentation. In various fermentation pathways, pyruvate serves as the electron acceptor, being reduced by NADH. This reduction step regenerates NAD+, allowing glycolysis to continue. This is the crucial step where NAD+ is regenerated under anaerobic conditions, the defining characteristic of fermentation.
-
End-Product Formation: The reduced pyruvate gives rise to the characteristic end-products of each fermentation type. These vary significantly and are used to classify different fermentation pathways.
Different Types of Fermentation: A Spectrum of Metabolic Strategies
Fermentation pathways are incredibly diverse. The diversity reflects the adaptability of microorganisms to exploit various niches and substrates. Here are some key examples:
-
Lactic Acid Fermentation: This pathway is used by bacteria (like Lactobacillus) and muscle cells under anaerobic conditions. Pyruvate is directly reduced to lactate, with NADH being oxidized to NAD+. This is responsible for the sour taste of yogurt and sauerkraut, and also contributes to muscle fatigue during intense exercise.
-
Alcoholic Fermentation: This pathway, famously used by yeast (Saccharomyces cerevisiae), converts pyruvate to ethanol and carbon dioxide. Acetaldehyde, an intermediate, acts as the electron acceptor, being reduced by NADH to ethanol. This process is responsible for the production of beer, wine, and bread.
-
Propionic Acid Fermentation: This pathway is carried out by propionic acid bacteria, resulting in the formation of propionic acid, acetic acid, and carbon dioxide. This fermentation is important in the production of Swiss cheese, contributing to its characteristic flavor and holes.
-
Butyric Acid Fermentation: Certain anaerobic bacteria produce butyric acid, butanol, acetone, and carbon dioxide through this pathway. This type of fermentation is often associated with the spoilage of food products.
-
Mixed Acid Fermentation: This pathway is characteristic of Escherichia coli and other enteric bacteria. It produces a mixture of acids, including lactic acid, acetic acid, succinic acid, formic acid, and ethanol.
The Scientific Explanation: Redox Reactions and Enzyme Catalysis
At the heart of fermentation lies a series of redox reactions – reactions involving the transfer of electrons. These reactions are precisely controlled by specific enzymes.
-
Dehydrogenase Enzymes: These enzymes catalyze the oxidation of NADH to NAD+ and the simultaneous reduction of pyruvate (or other intermediate molecules). Each fermentation pathway employs specific dehydrogenase enzymes tailored to the specific reaction.
-
Electron Transfer: The transfer of electrons from NADH to pyruvate (or its derivatives) is the key event. This transfer regenerates NAD+ and allows glycolysis to continue producing ATP, albeit at a reduced rate.
-
Energy Yield: While fermentation generates only 2 ATP molecules per glucose molecule (from glycolysis), it is crucial for survival under anaerobic conditions. It's significantly less efficient than aerobic respiration (which yields 36-38 ATP molecules), but it prevents a complete shutdown of energy production.
Industrial Applications of Fermentation: Beyond Beverages
The industrial applications of fermentation are vast and diverse, extending far beyond the production of alcoholic beverages. Fermentation is crucial in several industries:
-
Food Production: Fermentation is widely used in the production of yogurt, cheese, sauerkraut, kimchi, bread, and many other foods. It enhances flavor, texture, and preservation.
-
Pharmaceutical Industry: Fermentation is used to produce a wide range of pharmaceuticals, including antibiotics (e.g., penicillin), vitamins (e.g., riboflavin), and other bioactive compounds.
-
Biofuel Production: Fermentation processes are being explored and developed for the production of biofuels, such as ethanol from biomass.
-
Bioremediation: Certain microorganisms use fermentation to break down pollutants, playing a vital role in bioremediation efforts.
Frequently Asked Questions (FAQ)
-
Q: Is fermentation aerobic or anaerobic? A: Fermentation is strictly anaerobic. It occurs in the absence of oxygen.
-
Q: What is the main purpose of fermentation? A: The main purpose is to regenerate NAD+ from NADH, allowing glycolysis to continue and producing a small amount of ATP even in the absence of oxygen.
-
Q: What are the end products of fermentation? A: The end products vary depending on the type of fermentation, but common examples include lactic acid, ethanol, carbon dioxide, acetic acid, propionic acid, and butyric acid.
-
Q: Is fermentation efficient? A: Compared to aerobic respiration, fermentation is significantly less efficient in terms of ATP production. However, it provides a vital survival mechanism under anaerobic conditions.
-
Q: How does fermentation contribute to food preservation? A: The acidic end products of many fermentation processes inhibit the growth of spoilage microorganisms, contributing to food preservation.
Conclusion: A Fundamental Metabolic Process
Fermentation, the regeneration of NAD+ under anaerobic conditions, is a fundamental metabolic process with profound implications for microbiology, biotechnology, and our understanding of cellular energy production. While less efficient than aerobic respiration, it is a critical survival strategy for many organisms, allowing them to thrive in oxygen-deprived environments. Its diverse applications in food production, pharmaceuticals, and biofuel generation highlight its significant economic and societal importance. Continued research in this area promises to uncover further applications and deepen our comprehension of this essential metabolic pathway. Understanding the nuances of fermentation enhances our ability to harness its power for various beneficial applications while also appreciating its crucial role in the ecology and survival of diverse life forms on Earth.
Latest Posts
Latest Posts
-
5 Client Findings That Require Further Evaluation
Sep 10, 2025
-
In Our Pharmacy Which Of The Following Activities Are Allowed
Sep 10, 2025
-
Why Is The Production Of Sport Simulation Games Tricky
Sep 10, 2025
-
Suicide Starts To Become A More Serious Problem By
Sep 10, 2025
-
If You Discover A Data Breach
Sep 10, 2025
Related Post
Thank you for visiting our website which covers about The Regeneration Of ______ Under ______ Conditions Is Called Fermentation. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.