Temperature Is A Measure Of _________ Particles In An Object.
circlemeld.com
Sep 07, 2025 · 7 min read
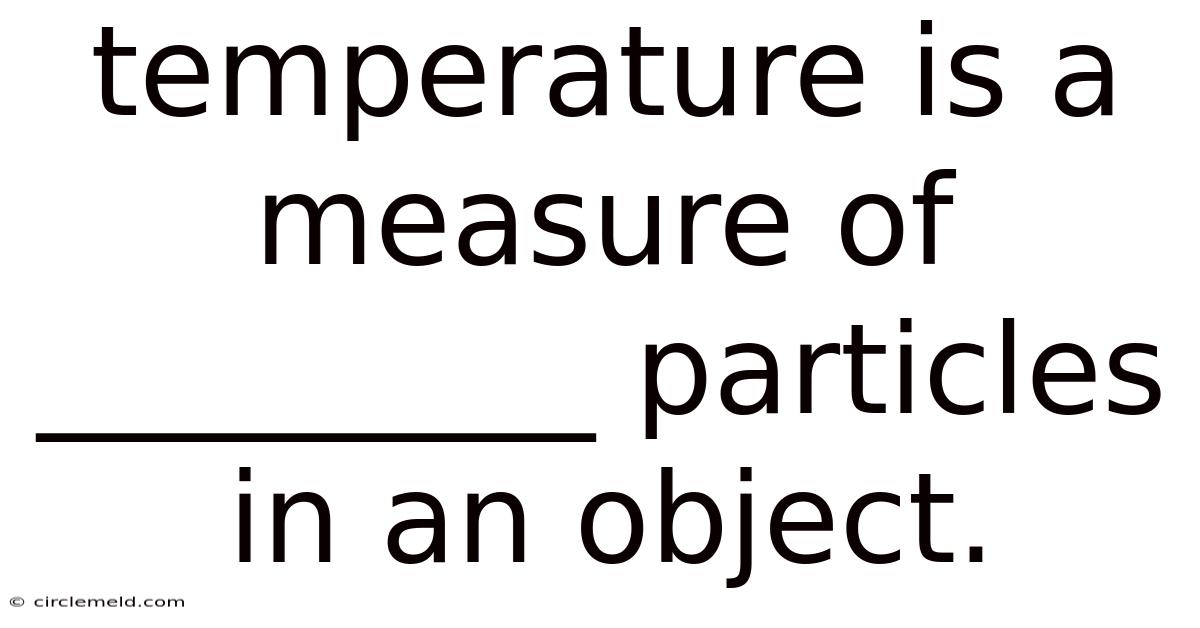
Table of Contents
Temperature: A Measure of the Average Kinetic Energy of Particles in an Object
Temperature is a fundamental concept in physics and everyday life. We experience it constantly, feeling the warmth of the sun, the chill of a winter's day, or the comforting heat of a fire. But what exactly is temperature? At its core, temperature is a measure of the average kinetic energy of the particles (atoms and molecules) in an object. This seemingly simple statement unlocks a wealth of understanding about the physical world, from the behavior of gases to the intricacies of thermodynamics. This article will delve deep into this definition, exploring its implications and providing a comprehensive understanding of temperature's significance.
Understanding Kinetic Energy
Before we dive into the relationship between temperature and kinetic energy, let's clarify what kinetic energy is. Kinetic energy is the energy an object possesses due to its motion. The faster an object moves, the greater its kinetic energy. This applies equally to macroscopic objects like cars and microscopic particles like atoms and molecules. These particles are in constant motion, even in seemingly stationary objects. They vibrate, rotate, and translate (move from place to place), contributing to their overall kinetic energy.
The Link Between Temperature and Kinetic Energy
The connection between temperature and kinetic energy is direct and proportional. A higher temperature means the particles within an object possess a higher average kinetic energy. Conversely, a lower temperature indicates a lower average kinetic energy. It's crucial to emphasize the word "average" here. Not all particles in an object will have the same kinetic energy at a given temperature. Some will be moving faster, some slower, but the temperature reflects the average kinetic energy of the entire ensemble.
Imagine a container filled with gas molecules. At a low temperature, these molecules move relatively slowly, colliding infrequently with each other and the container walls. As you increase the temperature, the molecules gain kinetic energy, moving faster and colliding more frequently and forcefully. This increased molecular motion is what we perceive as an increase in temperature.
Measuring Temperature: Scales and Units
Temperature is typically measured using various scales, including Celsius (°C), Fahrenheit (°F), and Kelvin (K). While Celsius and Fahrenheit are based on arbitrary reference points (the freezing and boiling points of water), Kelvin is an absolute scale. Zero Kelvin (0 K), also known as absolute zero, represents the theoretical point at which all molecular motion ceases. This is the lowest possible temperature, and it's a crucial reference point in many scientific calculations.
The Role of Heat Transfer
Heat transfer is the process by which thermal energy moves from one object or system to another. This transfer always occurs from a region of higher temperature to a region of lower temperature, until thermal equilibrium is reached (both objects are at the same temperature). This movement of thermal energy is directly related to the transfer of kinetic energy between particles. When a hot object comes into contact with a cold object, the faster-moving particles in the hot object collide with the slower-moving particles in the cold object, transferring some of their kinetic energy. This results in a decrease in the average kinetic energy of the hot object (lower temperature) and an increase in the average kinetic energy of the cold object (higher temperature).
Different States of Matter and Temperature
The state of matter (solid, liquid, or gas) of a substance is also closely linked to the average kinetic energy of its particles and therefore its temperature.
-
Solids: In solids, particles are tightly bound together and possess relatively low kinetic energy. They vibrate in place, but their movement is restricted. Increasing the temperature increases the vibrational energy, but the solid structure remains intact until the melting point is reached.
-
Liquids: In liquids, particles have more kinetic energy than in solids. They are less tightly bound and can move around more freely, though they are still relatively close together. Increasing the temperature further increases their kinetic energy and fluidity.
-
Gases: In gases, particles have the highest kinetic energy. They are far apart and move randomly at high speeds, colliding frequently with each other and the container walls. Increasing the temperature significantly increases their speed and the frequency of collisions.
The Microscopic View: Statistical Mechanics
A deeper understanding of temperature requires delving into statistical mechanics. This branch of physics uses probability and statistics to describe the behavior of large numbers of particles. It explains how macroscopic properties like temperature emerge from the microscopic interactions of countless individual particles. The average kinetic energy isn't simply an average of individual speeds but is connected to the distribution of speeds among particles, often described by the Maxwell-Boltzmann distribution. This distribution shows the probability of a particle having a specific kinetic energy at a particular temperature.
Temperature and Phase Transitions
Phase transitions, such as melting, boiling, and sublimation, are directly related to changes in the average kinetic energy of particles. The amount of energy required to cause a phase transition (latent heat) is a measure of the energy needed to overcome the intermolecular forces holding the particles together. For example, melting a solid requires adding enough energy to increase the average kinetic energy to the point where the particles can overcome the attractive forces holding them in a fixed lattice structure.
Beyond Average Kinetic Energy: Internal Energy
While temperature is directly related to the average kinetic energy of particles, it's not the only contributor to a system's total energy. Internal energy is the total energy contained within a system, encompassing both kinetic and potential energy. Potential energy arises from the interactions between particles (e.g., intermolecular forces). Changes in internal energy can result from heat transfer or work done on the system. While temperature is a good indicator of the kinetic component of internal energy, it doesn't fully capture the entire energy content of a system.
Applications of Temperature Measurement
Temperature measurement and control are crucial in countless applications across various fields, including:
-
Medicine: Monitoring body temperature is essential for diagnosis and treatment.
-
Industry: Precise temperature control is vital in manufacturing processes, such as metalworking, food processing, and chemical engineering.
-
Environmental Science: Temperature monitoring is essential for climate change research and weather forecasting.
-
Research: Accurate temperature control is critical in scientific experiments across various disciplines.
Frequently Asked Questions (FAQ)
Q: Can temperature be negative?
A: On the Celsius and Fahrenheit scales, negative temperatures are possible, indicating temperatures below the freezing point of water. However, on the Kelvin scale, negative temperatures are not possible because 0 K represents absolute zero. While there has been recent discussion and research on negative absolute temperatures, this is a specific situation in quantum systems and doesn't negate the fundamental definition of temperature as related to average kinetic energy.
Q: Does the mass of particles affect temperature?
A: While the kinetic energy of a particle is related to its mass and velocity (KE = 1/2mv²), temperature is a measure of average kinetic energy. Heavier particles will have a lower velocity at the same kinetic energy as lighter particles. Therefore, the temperature reflects the overall kinetic energy distribution regardless of individual particle masses.
Q: How does temperature affect the volume of a gas?
A: According to the ideal gas law (PV=nRT), temperature is directly proportional to the volume of a gas at constant pressure. Higher temperatures mean increased particle kinetic energy, leading to greater expansion and increased volume.
Q: Can two objects at different temperatures have the same total kinetic energy?
A: Yes, this is possible. The total kinetic energy depends on the number of particles as well as their average kinetic energy. A large object at a lower temperature could have a higher total kinetic energy than a small object at a higher temperature. Temperature measures the average kinetic energy per particle, not the total kinetic energy.
Conclusion
Temperature, at its fundamental level, is a direct measure of the average kinetic energy of the particles within an object. This seemingly simple concept opens doors to a deep understanding of the physical world, from the behavior of individual atoms and molecules to the macroscopic properties of matter and the processes of heat transfer and phase transitions. Understanding the relationship between temperature and kinetic energy provides a crucial foundation for comprehending a wide range of scientific phenomena and technological applications. The exploration of temperature goes beyond a simple definition, leading us into the intricacies of statistical mechanics, thermodynamics, and the diverse ways temperature influences our world.
Latest Posts
Latest Posts
-
To Avoid Exacerbating A Patients Injury Quizlet
Sep 08, 2025
-
Quizlet Anatomy And Physiology Chapter 4
Sep 08, 2025
-
Quizlet The Great Gatsby Chapter 7
Sep 08, 2025
-
What Is An Element Of Performance For The National Quizlet
Sep 08, 2025
-
By Law Your Institution Prohibits Quizlet
Sep 08, 2025
Related Post
Thank you for visiting our website which covers about Temperature Is A Measure Of _________ Particles In An Object. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.