Atomic Radius _______ From Left To Right Across A Period
circlemeld.com
Sep 06, 2025 · 6 min read
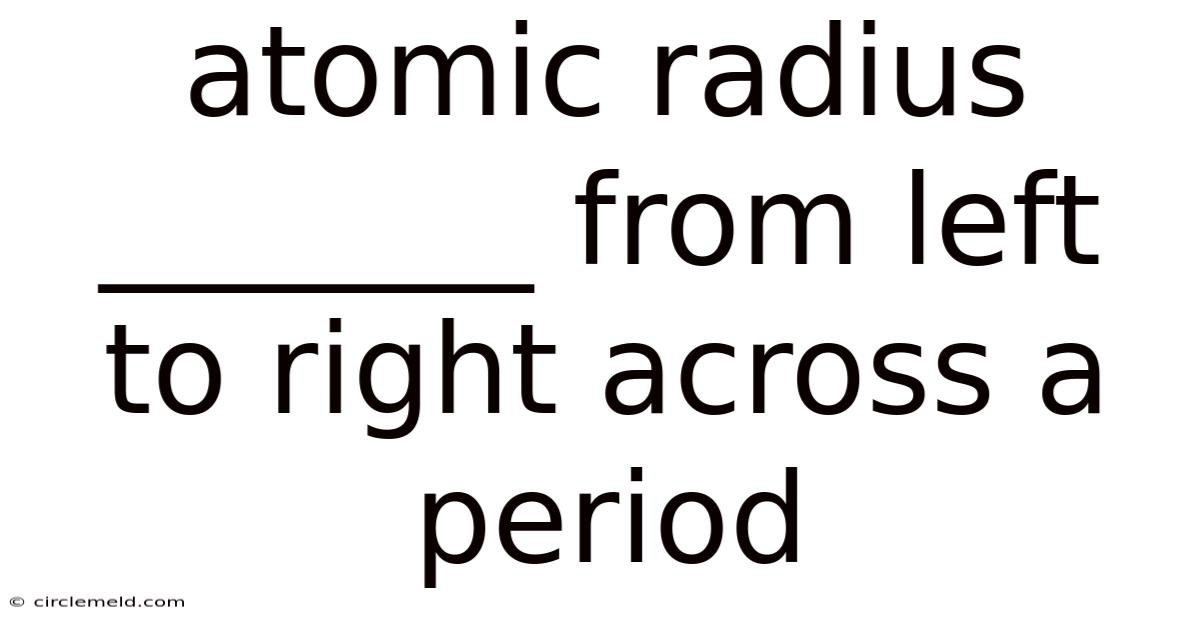
Table of Contents
Atomic Radius: Decreasing Across a Period – A Deep Dive into Periodic Trends
Understanding atomic radius is fundamental to grasping the behavior of elements and their interactions. This article delves into the fascinating trend of decreasing atomic radius from left to right across a period in the periodic table. We'll explore the underlying reasons, provide clear explanations, and address common misconceptions. This in-depth analysis will equip you with a comprehensive understanding of this crucial periodic trend.
Introduction: The Periodic Table and its Trends
The periodic table organizes elements based on their atomic number and recurring chemical properties. One of the most significant periodic trends is the change in atomic radius. Atomic radius refers to the distance from the atom's nucleus to its outermost electron shell. While seemingly simple, understanding how and why this radius changes is key to predicting chemical reactivity and other elemental behaviors. This article specifically focuses on the consistent decrease in atomic radius observed when moving from left to right across a period (a horizontal row) in the periodic table.
Factors Affecting Atomic Radius
Several factors interplay to determine an atom's size. The most important are:
- Nuclear Charge: The number of protons in the nucleus. A higher nuclear charge exerts a stronger positive pull on the electrons.
- Shielding Effect: The inner electrons partially shield the outer electrons from the full positive charge of the nucleus. Inner electrons repel outer electrons, reducing the effective nuclear charge felt by the outermost electrons.
- Electron-Electron Repulsion: Outer electrons repel each other, slightly increasing the atomic radius.
Why Atomic Radius Decreases Across a Period
As we move across a period from left to right, the atomic number increases, meaning more protons are added to the nucleus. This leads to a stronger positive nuclear charge. Simultaneously, within a period, electrons are added to the same principal energy level (shell). The increased nuclear charge pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
While electrons are added to the same shell, the shielding effect remains relatively constant across a period. The additional electrons are added to the outermost shell, and the inner electrons provide roughly the same level of shielding. Therefore, the dominant effect is the increased nuclear charge, which outweighs the slight increase in electron-electron repulsion. This ultimately leads to a consistent decrease in atomic radius.
In essence: The stronger pull from the increasing number of protons in the nucleus dominates over the increased electron-electron repulsion and relatively constant shielding effect, resulting in a smaller atomic radius.
Visualizing the Trend
Imagine a balloon (the electron cloud) being pulled inwards by increasingly strong strings (the nuclear charge) while the number of strings increases but the balloon's size (energy level) remains the same. This analogy illustrates the decrease in atomic radius across a period.
Exceptions and Nuances
While the general trend of decreasing atomic radius across a period is consistent, there can be minor exceptions or variations. These deviations often arise due to electron configurations and subtle interactions within the electron shells. For instance, some elements might exhibit slightly larger or smaller radii due to variations in electron-electron repulsion or subtle changes in shielding. However, the overall trend remains largely consistent.
Detailed Explanation with Examples
Let's consider Period 3 (Sodium to Argon) to illustrate this trend:
- Sodium (Na): Has 11 protons and 11 electrons. Its outermost electron is relatively far from the nucleus.
- Magnesium (Mg): Has 12 protons and 12 electrons. The increased nuclear charge pulls the electrons slightly closer.
- Aluminum (Al): Continues the trend with 13 protons and 13 electrons.
- Silicon (Si): With 14 protons, the trend persists.
- Phosphorus (P): The increasing nuclear charge continues to contract the atom.
- Sulfur (S): Shows a further decrease in atomic radius.
- Chlorine (Cl): Exhibits a continued decrease.
- Argon (Ar): Has the smallest atomic radius in Period 3, reflecting the maximum nuclear charge in this period.
The incremental increase in nuclear charge across Period 3, with electrons added to the same shell, results in a systematic decrease in atomic radius from sodium to argon.
The Significance of Atomic Radius
The atomic radius significantly influences various chemical and physical properties of elements. Some key implications are:
- Chemical Reactivity: Smaller atoms have a greater tendency to attract electrons, often leading to higher electronegativity and a greater likelihood of forming chemical bonds.
- Melting and Boiling Points: Atomic radius influences interatomic forces, affecting the energy required to change the state of matter.
- Density: Smaller atomic radius often translates to higher density, as more atoms can pack into a given volume.
- Ionization Energy: It requires more energy to remove an electron from a smaller atom because the electron is more strongly bound to the nucleus.
Frequently Asked Questions (FAQ)
Q1: Does atomic radius always decrease across a period?
A1: While the general trend is a decrease, minor exceptions exist due to electron configurations and subtle inter-electron interactions. The overall trend, however, remains highly consistent.
Q2: How is atomic radius measured?
A2: Atomic radius is not a directly measurable quantity like length. It's determined through various experimental techniques and theoretical calculations, such as X-ray diffraction and spectroscopic methods, which provide information about interatomic distances in solids and molecules. These data are then used to infer atomic radii.
Q3: What is the difference between atomic radius and ionic radius?
A3: Atomic radius refers to the size of a neutral atom, while ionic radius refers to the size of an ion (a charged atom). Anions (negatively charged ions) are generally larger than their corresponding neutral atoms, while cations (positively charged ions) are smaller.
Q4: How does atomic radius relate to other periodic trends?
A4: Atomic radius is intrinsically linked to other periodic trends, such as ionization energy (the energy needed to remove an electron), electronegativity (the ability to attract electrons), and electron affinity (the energy change associated with gaining an electron). These trends are all interconnected and influenced by the same fundamental factors – nuclear charge, shielding effect, and electron-electron repulsion.
Conclusion: A Fundamental Periodic Trend
The decrease in atomic radius across a period is a fundamental periodic trend with far-reaching consequences for the properties and behavior of elements. Understanding this trend is crucial for predicting chemical reactivity, bonding patterns, and physical properties. The interplay between nuclear charge, shielding, and electron-electron repulsion provides a comprehensive explanation for this observation, highlighting the elegant order and predictability within the periodic table. By grasping this concept, we unlock a deeper understanding of the intricate relationships between the elements and their remarkable diversity. Further exploration of this topic can delve into the nuances and exceptions, providing a more complete picture of the periodic table's structure and functionality.
Latest Posts
Latest Posts
-
Which Information Would The Nurse Provide A Sutdent About Floating
Sep 07, 2025
-
What Is The Difference Between Covalent Bonds And Ionic Bonds
Sep 07, 2025
-
The Number Of Cells Produced In Meiosis Is
Sep 07, 2025
-
Which Of The Following Is True Regarding Research Misconduct
Sep 07, 2025
-
Explain How Western Society Changed During The Cold War Era
Sep 07, 2025
Related Post
Thank you for visiting our website which covers about Atomic Radius _______ From Left To Right Across A Period . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.